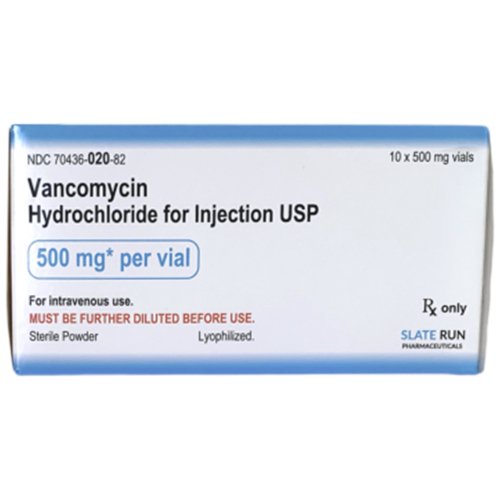
Vancomycin HCl 500mg Injectable Powder
AND-70436002082
Slate Run
-
RX License on File Required to Order
Detail
Antibiotics
Product Overview:
Vancomycin HCl 500mg Injectable Powder is a sterile, lyophilized powder intended for reconstitution and intravenous administration. It is an antibiotic used to treat serious or severe bacterial infections that are resistant to other antibiotics, particularly methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive infections.
Key Features:
- Potent Antibiotic Action:
- Vancomycin is known for its efficacy against a wide range of Gram-positive bacteria, including MRSA, Streptococcus species, and Enterococcus species. It inhibits cell wall synthesis, leading to bacterial cell death.
- Vancomycin HCl 500mg Injectable Powder is indicated for the treatment of severe bacterial infections where other antibiotics are not effective. This includes infections such as endocarditis, osteomyelitis, pneumonia, and sepsis caused by susceptible organisms.
- The powder is reconstituted with sterile water or another suitable diluent to prepare a solution for intravenous infusion. The dosage and frequency are determined by the severity of the infection, patient’s age, weight, renal function, and clinical response.
- The product is supplied as a 500mg vial of vancomycin hydrochloride powder. Once reconstituted, it should be used promptly to ensure potency and minimize the risk of contamination.
- The powder is produced in a sterile environment and is free from pyrogens, ensuring safe administration to patients, particularly those in critical care.
Indications for Use:
- Severe Infections: Vancomycin is particularly effective for treating infections caused by MRSA and other resistant Gram-positive bacteria. It is often used when other antibiotics fail or are contraindicated.
- Empiric Therapy: It is also used as part of empiric therapy when a serious bacterial infection is suspected, and resistant organisms are a concern.
Administration Guidelines:
- Reconstitution: Reconstitute the 500mg vial with an appropriate amount of sterile water or diluent to achieve the desired concentration. Ensure complete dissolution before administering.
- Infusion: The reconstituted solution is administered as a slow intravenous infusion, typically over 60 minutes or more, to reduce the risk of infusion-related reactions, such as "red man syndrome."
- Dosage: The dosage should be adjusted based on the patient’s renal function and the severity of the infection. Therapeutic drug monitoring (TDM) may be required to optimize dosing.
Contraindications and Precautions:
- Contraindications: Hypersensitivity to vancomycin or any component of the formulation.
- Precautions: Use with caution in patients with renal impairment, as vancomycin is primarily excreted by the kidneys. Monitor renal function and adjust the dosage as needed. Be vigilant for signs of ototoxicity, particularly in patients with pre-existing hearing loss.
Potential Side Effects:
- Common: Red man syndrome (a rate-dependent infusion reaction characterized by flushing, rash, and hypotension), phlebitis, and nephrotoxicity.
- Serious: Ototoxicity, particularly with high doses or prolonged therapy, and neutropenia.
Storage and Stability:
- Before Reconstitution: Store the vial at room temperature, away from light and moisture.
- After Reconstitution: The reconstituted solution should be used immediately. If necessary, it may be stored under refrigeration but should be used within the time frame specified in the product labeling.

 0
0