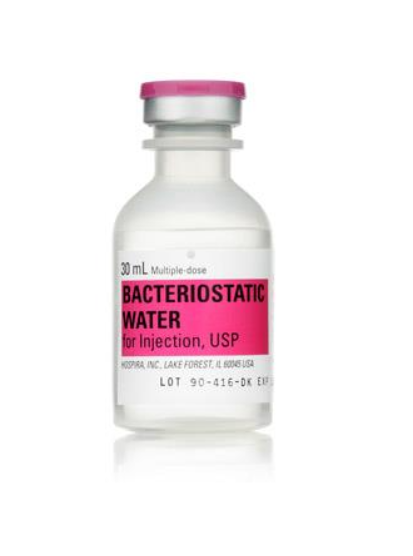
Sodium Chloride 0.9% | 30mL Bacteriostatic Injectable Fliptop MDV
Pfizer
-
RX License on File Required to Order
Detail
The Sodium Chloride 0.9% Bacteriostatic Solution in a 30mL Fliptop Multi-Dose Vial (MDV) is a sterile, isotonic solution intended for intravenous administration. It contains 0.9% sodium chloride and 0.9% benzyl alcohol as a bacteriostatic agent to inhibit bacterial growth. This product is commonly used for diluting medications or as a vehicle for drug administration, ensuring sterility and patient safety.
Features and Benefits
- Sterile and Bacteriostatic: The addition of benzyl alcohol acts as a preservative, allowing for multiple withdrawals while maintaining sterility.
- Convenient Fliptop Vial: The fliptop cap provides easy access and resealing, ensuring the solution remains sterile between uses.
- Multi-Dose Capability: Designed for multiple withdrawals, it offers flexibility and efficiency in medication preparation and administration.
- Isotonic Solution: Matches the osmolarity of body fluids, making it safe for intravenous administration without causing fluid imbalance.
- Versatile Use: Ideal for diluting medications, reconstituting powdered drugs, and flushing IV lines.
Indications
- Medication Dilution: Used to dilute medications for intravenous, intramuscular, or subcutaneous injection.
- Drug Reconstitution: Reconstitutes powdered medications for injection or infusion.
- IV Line Flushing: Maintains patency of IV catheters and lines, preventing clot formation.
Administration
- Intravenous Administration: Administered directly into the vein, either alone or as a diluent for other medications.
- Dosage: The volume and rate of administration should be determined by the healthcare provider, based on the specific medication being diluted and the patient’s condition.
- Storage: Store at controlled room temperature. Avoid freezing and excessive heat to maintain product integrity.
Precautions
- Allergic Reactions: Monitor for signs of allergic reactions to benzyl alcohol, though such instances are rare.
- Electrolyte Imbalance: Regularly monitor electrolytes to avoid potential imbalances, especially in patients with renal or cardiovascular issues.
- Proper Use: Ensure aseptic technique during withdrawal to maintain sterility. Do not use if the solution is cloudy or contains particulates.
Composition
- Active Ingredient: Sodium Chloride 9 g/L
- Preservative: Benzyl Alcohol 9 mg/mL
- Water for Injection: QS to 30 mL
- Osmolarity: Approximately 308 mOsm/L
- pH: 4.5-7.0
Packaging
The Sodium Chloride 0.9% solution is packaged in a 30mL fliptop multi-dose vial. The vial is designed for multiple withdrawals, with a bacteriostatic agent ensuring sterility between uses.
Regulatory Status
- Compliance: Meets USP standards for sterile solutions.
- Approval: FDA-approved for intravenous administration and multi-dose usage.
This Sodium Chloride 0.9% Bacteriostatic Solution in a 30mL Fliptop MDV is a versatile and essential product for medication preparation and administration in medical settings. Its bacteriostatic property, combined with the convenience of a fliptop vial, ensures sterility and flexibility, making it a valuable asset in clinical practice. Always consult healthcare professionals for proper usage and administration guidelines.

 0
0