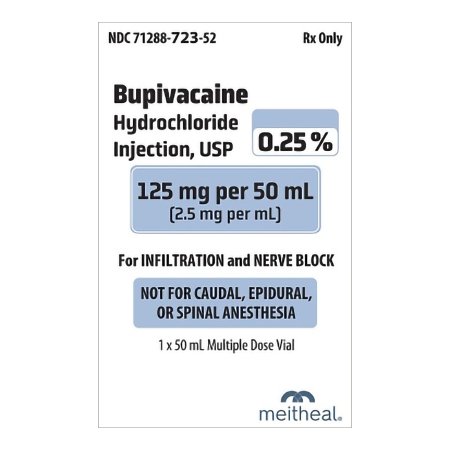
Bupivacaine 0.25% | 2.5 mg | 50mL Injectable MDV
Meitheal Pharmaceuticals
-
RX License on File Required to Order
Detail
Bupivacaine Hydrochloride Injection, USP 0.25% (2.5 mg/mL) is a sterile, nonpyrogenic, aqueous solution intended for parenteral administration via injection. Each 50 mL multiple-dose vial contains 125 mg of bupivacaine hydrochloride. This product is manufactured by Meitheal Pharmaceuticals and is equivalent to the brand name Marcaine.
Indications and Usage: Bupivacaine is a local anesthetic commonly used to numb specific areas of the body during surgical procedures, childbirth, dental work, or other medical interventions. It works by blocking nerve signals in the body, thereby reducing pain sensation.
Dosage and Administration: The concentration of 0.25% (2.5 mg/mL) is suitable for various types of local and regional anesthesia. The specific dosage and administration route should be determined by a healthcare professional based on the type of procedure, the area to be anesthetized, and the patient's condition.
Packaging and Availability: This product is supplied in 50 mL multiple-dose glass vials with a 20 mm closure. Each unit of sale includes one pack containing a single vial. The minimum order quantity is one case, which consists of 50 packs.
Safety Information: Bupivacaine Hydrochloride Injection is not made with natural rubber latex and is AP rated. As with all local anesthetics, it should be administered by qualified healthcare professionals familiar with its properties and safe usage. Proper resuscitative equipment and medications should be readily available during administration. Patients should be monitored for signs of systemic toxicity, especially when large doses are administered or when used in areas with a high degree of vascularity.
Storage Conditions: Store at controlled room temperature as specified in the product's package insert. Protect from light and do not freeze.
For more detailed information, including potential side effects, contraindications, and drug interactions, please refer to the full prescribing information provided by Meitheal Pharmaceuticals

 0
0

![[PFZ-00409156029] Marcaine™ (Bupivacaine) 0.5% | 5mg | 30mL Injectable SDV](/web/image/product.template/11465/image_512/%5BPFZ-00409156029%5D%20Marcaine%E2%84%A2%20%28Bupivacaine%29%200.5%25%20%7C%205mg%20%7C%2030mL%20Injectable%20SDV%20?unique=a01d615)
